A 66 years old lady pseudophakic presented to me with features of ARMD. Her BCVA is 20/200 in her right eye, while in her left eye, the BCVA is 20/35. Fundus examination showed drusen and signs of ARMD.
Fundus image
Fundus photograph shows soft drusen, RPE atrophy, heterogeneous drusenoid formation presenting regressing (transforming) drusen, and adjacent to it, there is a small intraretinal cyst.
 |
| Fundus image showing regressing (transforming) drusen and RPE atrophy |
Optical coherence tomography
OCT cross-section shows RPE atrophy areas, which feature the absence of the RPE layer with pronounced Bruch’s membrane, and the light is not reflected by the RPE and passes through the choroid, increasing its reflectivity. Along with the presence of drusens and disruption of the ellipsoid zone with normal central retinal thickness.
 |
| OCT cross-section showing geographic atrophy adjacent to regressing (transforming) drusen |
Another finding of radial OCT cross-section is an intraretinal cyst; however, the raster cross-section shows heterogeneous sub RPE deposits forming regressing (transforming) drusen.
 |
| OCT cross-section showing geographic atrophy adjacent to intraretinal cyst and ellipsoid zone disruption |
Optical coherence tomography angiography
Shows absence of signal of neovascular network ruling out CNVm; however, there is choriocapillaris flow deficit in the area of drusen.
 |
| OCTa ruling out CNV network but featuring choriocapillaris flow defect in drusen area |
Fluorescein fundus angiography
Regressing (transforming) drusen feature heterogeneous hyperfluorescence that is more pronounced in the mid phases causing staining in late stages.
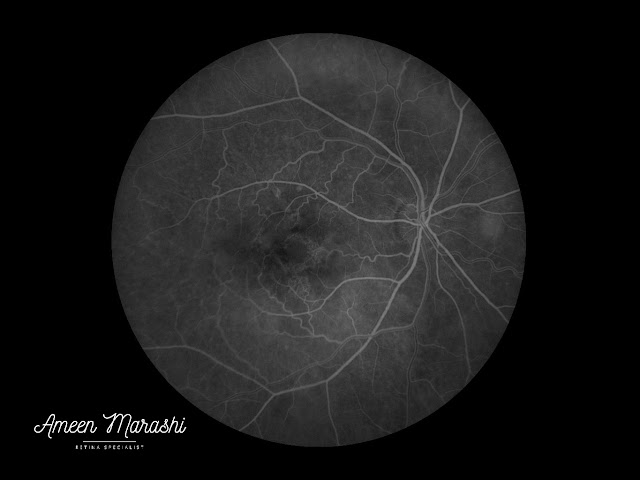 |
| Early phase FFA showing heterogeneous hyperfluorescence |
In contrast, to soft drusen where staining is homogeneous, along with hyperfluorescence in mid phases. The FFA does not show any signs of choroidal neovascularization.
 |
| Mid phase FFA showing heterogeneous hyperfluorescence from regressing (transforming) drusen and homogenous hyperfluorescence from soft drusen |
FFA shows an area of window defect which appears as increased hyperfluorescence from RPE atrophic patch without a leak or increase in size in the late phases.
 |
| Late phase FFA showing heterogeneous hyperfluorescence from regressing (transforming) drusen and staining from soft drusen |
Diagnosis is a case of regressing (transforming) drusen in the setting of non-neovascular advanced ARMD
Management close follow up with supplements
Discussion
Regressing (transforming) drusen is a heterogeneous content, usually a sign of drusen becoming calcified.
It varies in size and usually a prognostic factor of developing choroidal neovascularization or atrophy. In this case, the ellipsoid zone is disrupted, causing a reduction of vision, and there is a marked RPE atrophy.
Intraretinal cyst, which is located in the outer nuclear layer, is not causing macular edema with a lack of the presence of CNVm.
Intraretinal cysts can be unique (As in this case) or multiple, and usually, they are associated with geographic atrophy as a result of Muller cell degeneration or loss in an intermediate stage of the atrophic process.
It is essential to differentiate Intraretinal cysts in advanced ARMD from outer retinal tubulation, which presents in SD-OCT as a circular or focal structure in outer retinal layers with hyperreflective border contains hyper-reflective material.
Wet ARMD or Retinal Angiomatous Proliferation can differentiate from intraretinal cystic changes as the former may cause retinal thickening, sub retina fluid, subretinal hyperreflective material or/and pigment epithelial detachment with the presence of leak on FFA from CNVm
A take-home message
When regressing (transforming) drusen is diagnosed, a close follow-up is warranted as this type of drusen may be a prognostic factor for developing choroidal neovascularization or RPE atrophy.
Hence patient education is essential as intraretinal cysts without causing cystoid macular edema in the settings of advanced ARMD should be interpreted with caution, especially in the absence of fibrovascular pigment epithelial detachment, subretinal hyperreflective material, or leak from CNVm based on FFA.
CNVm can be ruled out using FFA, ICG, or OCTa because those cysts do not warrant any treatment in the absence of CNVm.
Please tell me how you would manage this case in your clinical practice??!!!
Comments
Post a Comment